|

On eBay Now...
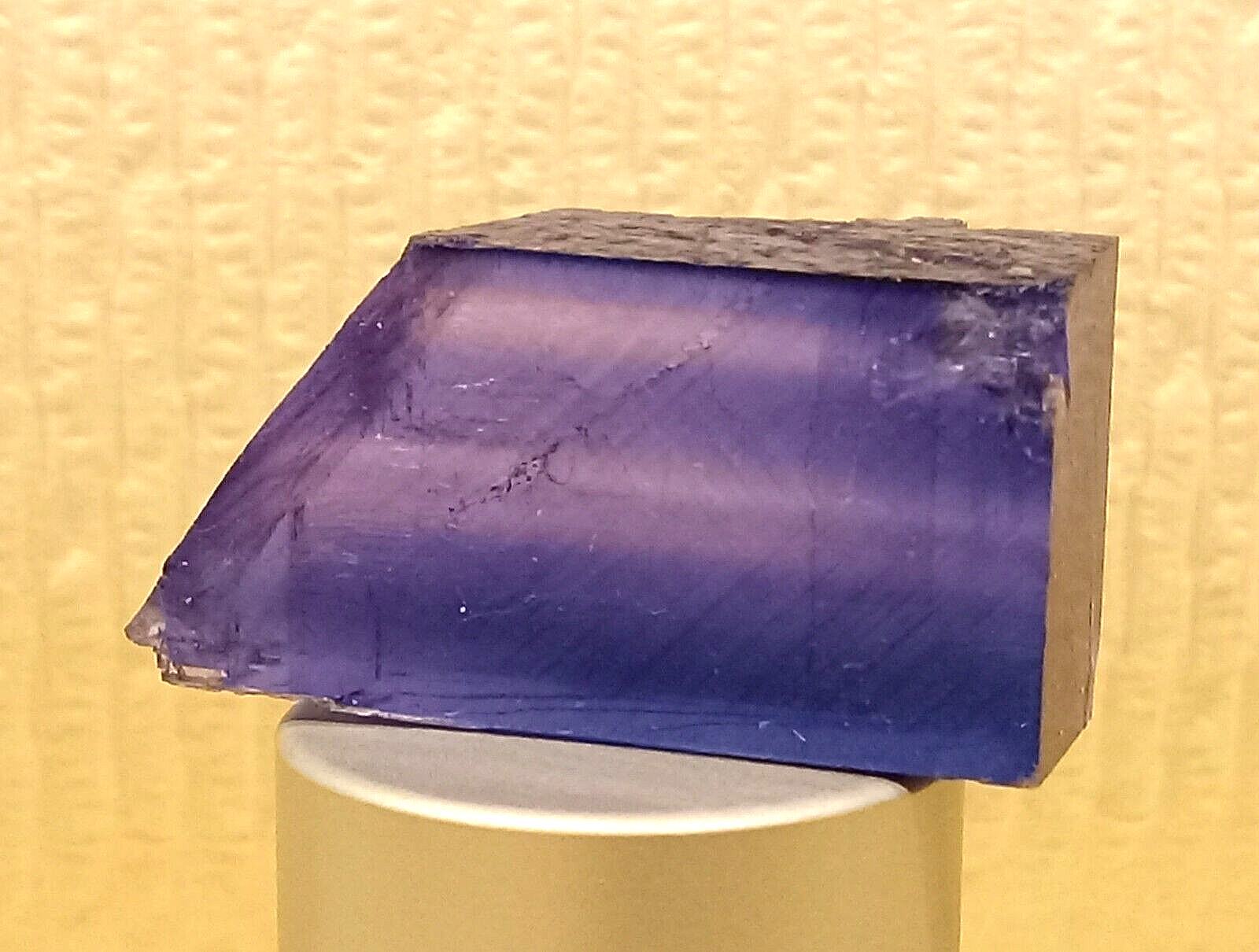
LUSTROUS BRIGHT VIVID BLUE HALITE CRYSTAL 1.2\" Transparent 7.1 grams For Sale
When you click on links to various merchants on this site and make a purchase, this can result in this site earning a commission. Affiliate programs and affiliations include, but are not limited to, the eBay Partner Network.

LUSTROUS BRIGHT VIVID BLUE HALITE CRYSTAL 1.2\" Transparent 7.1 grams:
$69.00
BRIGHT VIVID BLUE HALITE CRYSTAL<<<
\"Truly Beautiful Rare Gem Crystal\"Weight approx: 7.1 grams or 35.5 carats
Dimensions Length & Width approx: 29.15 mm X 15.0 mm X 11.15 mm (thick)
Dimensions Length & Width approx: 1.2\" X 0.6\" X 0.4\" (thick)
Origin: Carlsbad, Eddy County, New Mexico, USA.
Color: bright, vivid, blue.
Clarity: transparent & clear.
BLUE HALITE GEM CRYSTAL IS EXTREMELY LUSTROUS & SHINY..Makes a perfect & prized addition to your collection.
Utilize for metaphysical purposes,healing.
Truly a magnificent specimen.
Photos don\'t do justice.
DISCOUNTED PRICE: $189.
Includes draw string pouch for safe storage & FAST FREE SHIPPINGVia: USPS with tracking number & insurance.
LIMITED TIME ONLY: $69.WE\'RE OVERSTOCKED. DRASTICALLY REDUCING Due to the color contrast between different computer monitors, the photos may notreflectthe exact color of the item. Ladies & Gentlemen, if you\'re happy with your order, please leave us positive response. If you\'re not 100% satisfied,contact us & we will do everything possible to make your experience perfect. We sincerely appreciate your INFORMATION:
Halite belongs to the group of \"Halides\" which are essentially compounds in which metallic elements combine with halogens (which are bromine, chlorine, fluorine & iodine). Halite is found in sedimentaryrock that is formed,by the evaporation of water. So, it\'s considered an evaporite mineral due to the fact that it\'sformed by precipitation.In addition, also found in bedded sedimentary deposits.
Cleavage: perfect cubic
Crystal System: cubic
Fracture: uneven to conchoidal
Habit: cubic crystals
Hardness: 2.5
Specific Gravity: 2.1-2.2
Possesses a \"salty\" taste.
Soluble in cold water.
Feels \"greasy\" to the touch.
Luster: vitreous.
Clarity: transparent to translucent.
It turns a flame yellow in color.
Minute impurities will produce fluorescence colors of green, orange or red.
Halite lowers the freezing point of


Vanadinite Lustrous Bright Red Crystals On Matrix Morocco 12.5 Cms :} $88.99

Vanadinite Lustrous Bright Red Crystals On Matrix Morocco 3.1 Cms :} $44.99

LUSTROUS BRIGHT VIVID BLUE HALITE CRYSTAL 1.2" Transparent 7.1 grams $69.00

RARE,FINE,LUSTROUS BRIGHT SILVER ARSENOPYRITE CRYSTALS,PINK DOLOMITE, KOSOVO $200.00

Vanadinite Lustrous Bright Red Crystals Morocco 3.9 Cms :} $35.99

RARE,GORGEOUS,LUSTROUS BRIGHT YELLOW BRUCITE CRYSTALS,PAKISTAN $250.00

New Natural Lustrous Bright, Transparent Red Calcite Mineral Cluster Sample $40.00

Rare Frederick Cooper vintage Bronze metal lattice table lamp dragonfly bees 29" $135.00
|